Fuel cells for heavy commercial vehicles

The heart of every application
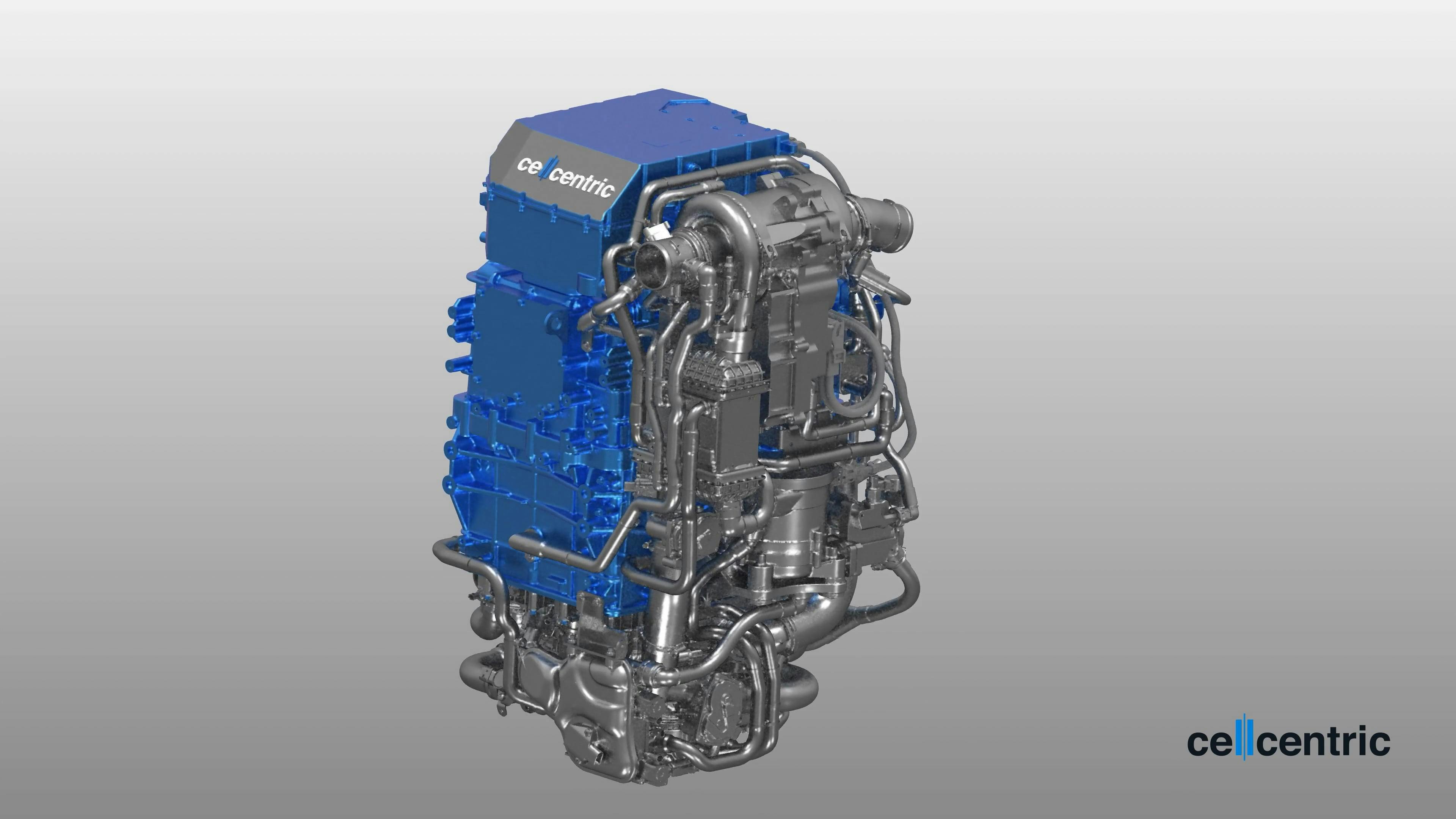
Our fuel cell systems are designed for use in heavy-duty commercial vehicles and other applications, developed accordingly to stringent automotive industry processes and standards for mass production. Therefore, setting the industry benchmark in cost, efficiency, durability and lifetime is the core concept of the development work of cellcentric.
You must accept marketing cookies in order to play the video.
We work on next-gen fuel cell systems
cellcentric does not simply scale up the previous passenger car technology to higher performance, but rather builds up a well-adapted system specifically for the demanding specifications of heavy-duty truck use.
The scalable fuel cell system has an output of approximately 150 kW and works at a voltage in the range of 800 VDC, is low weight with automotive-grade quality, and allows for quick refueling times. The geometry of the unit is based on the usual installation spaces of diesel engines, so that two units can be installed in the engine compartments of heavy-duty commercial vehicles with a total output of approximately 300 kW.
A close collaboration between the engineering and production teams ensures the high quality customers expect at a competitive price point due to the high volumes. Applying cellcentric’s technology to additional, non-automotive use cases at an early point in time creates additional benefits for all customers in product maturity and cost position.
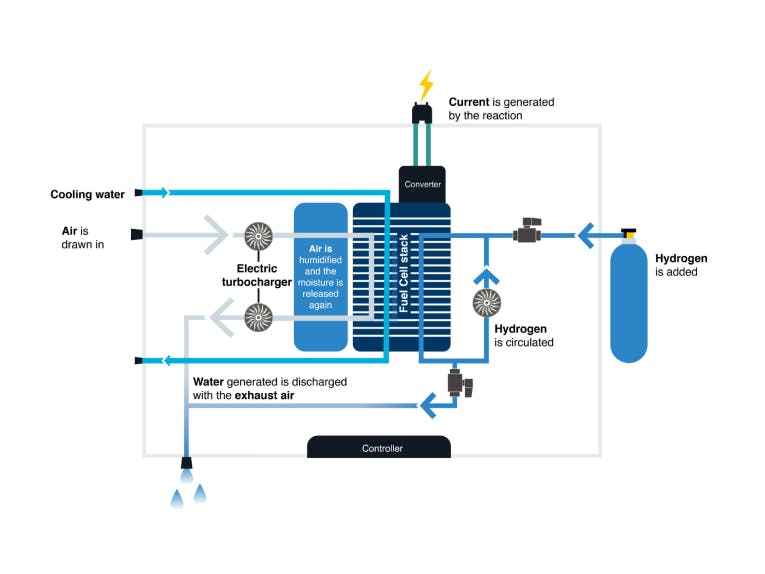
How a fuel cell system works
In a fuel cell, the continuously supplied hydrogen reacts with the oxidant oxygen. This produces water, electricity and heat. This electrochemical reaction is also called “cold combustion” and is particularly efficient.
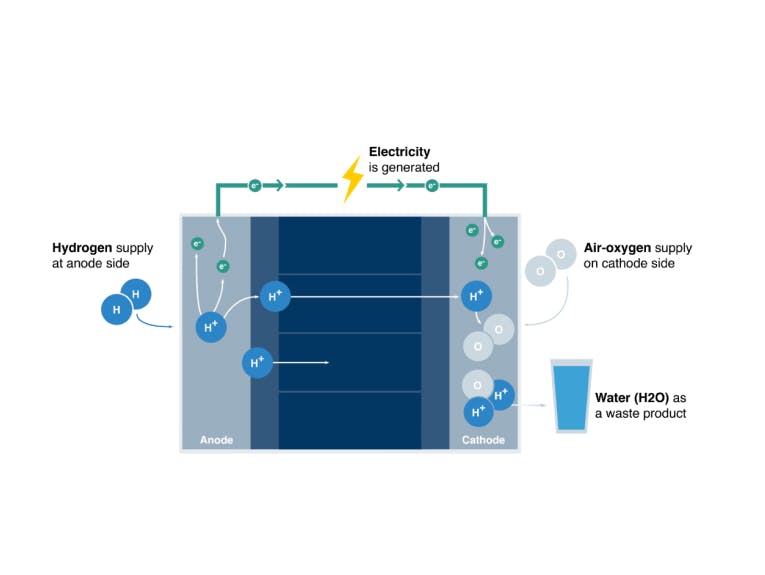
How a Fuel Cell Works
The systems developed by cellcentric are based on PEM (Proton Exchange Membrane) fuel cells, which are operated using hydrogen and oxygen from the air.
Function of a fuel cell
- Hydrogen is supplied from the anode side, atmospheric oxygen from the cathode side.
- The hydrogen molecule H2 is split into two protons and two electrons via a platinum catalyst.
- Electrons flow as electrical current through various electrical consumers, while the protons diffuse through the membrane.
- The potential difference between the anode and cathode generates electrical voltage. By stacking several cells, the voltage of several cells can be combined.
- The oxygen molecule O2 is split catalytically at the cathode into two oxygen ions through the acceptance of four electrons. Every oxygen ion connects with two protons to form a water molecule.
- The water is carried out of the fuel cell by the airflow.

Get in touch with us
Are you as excited about hydrogen and fuel cell technology as we are? Do you want to learn more about our work at cellcentric? We are here to help.
Contact us